Learn when in vivo and in vitro bioequivalence testing are used for generic drugs. Understand FDA requirements, cost differences, and which method works best for different drug types.
Category: Medicine and Pharmaceuticals - Page 4
Nasal congestion from overusing decongestant sprays is common and reversible. Learn how to break the cycle with proven methods like corticosteroids and saline rinses - and avoid relapse.
Pill packs and blister packaging help seniors take medications correctly by organizing doses by day and time. These systems reduce errors, improve adherence, and give independence to older adults managing multiple prescriptions.
Understand when to report serious vs non-serious adverse events in clinical trials. Learn the FDA and ICH criteria, reporting timelines, common mistakes, and how to avoid costly errors in safety monitoring.
Methadone can dangerously prolong the heart's QT interval, especially when combined with other medications. This article explains how the interaction works, who's at highest risk, and what steps can prevent sudden cardiac death.
Learn how generic drug substitution laws vary across all 50 U.S. states and D.C., including mandatory rules, patient consent, biosimilar requirements, and real-world impacts on patients and pharmacists.
Learn how pre-medication with steroids and antihistamines reduces contrast dye reaction risks from 35% to just 2%. Know who needs it, what protocols work, and how to stay safe during CT scans and X-rays.
Pricing pressure and rising manufacturing costs are causing widespread drug shortages as generic drug makers can't profitably produce essential medicines. Here's how the system broke - and what needs to change.
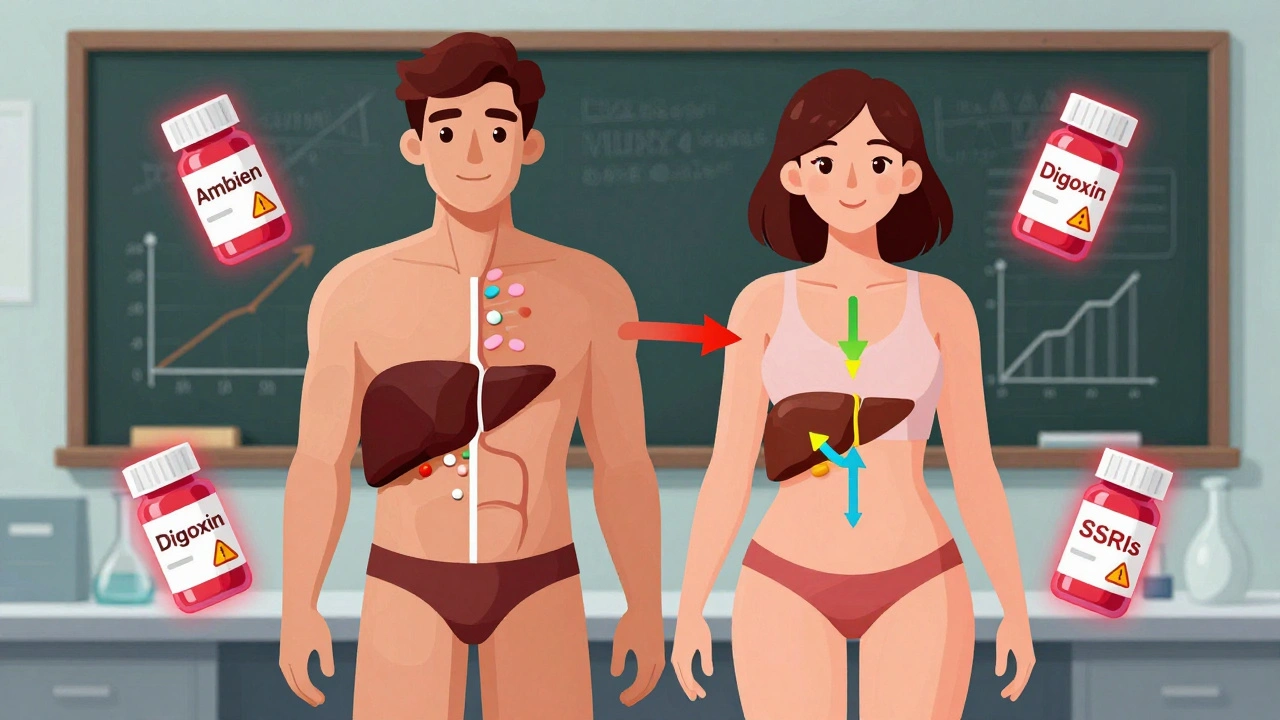
Women experience adverse drug reactions nearly twice as often as men due to biological differences and outdated clinical trial practices. Learn why dosing based on male physiology puts women at risk - and what’s being done to fix it.
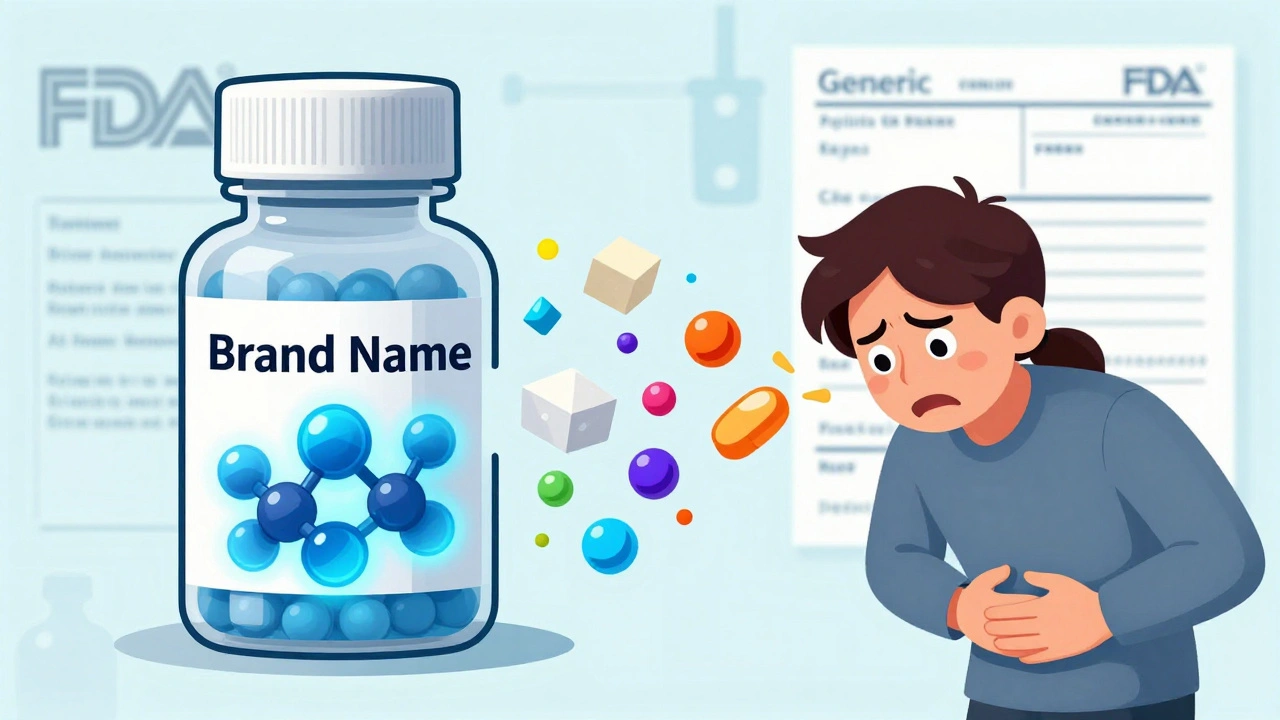
Generics are just as safe as brand-name drugs, but unexpected side effects can come from inactive ingredients-not the active drug. Learn what really causes interactions and how to stay safe.