Medication Dose Adjuster for Women
Adjust your dose based on biological differences
This tool provides general guidance only. Always consult your healthcare provider before changing medication.
Women are nearly twice as likely as men to have a bad reaction to the same dose of a medication. It’s not just in their head. It’s not because they’re more sensitive. It’s because most drugs were tested mostly on men - and women’s bodies process them differently.
Why Women Get More Side Effects
The numbers don’t lie. According to FDA data, women report adverse drug reactions 80% to 90% more often than men. That’s not a small gap. That’s a system failure. And it’s rooted in history. Back in the 1970s, the FDA told researchers to exclude women of childbearing age from early drug trials - not because it was scientifically sound, but to protect potential fetuses. The logic was well-intentioned, but the result was decades of data built on male bodies alone. Even after the 1993 NIH Revitalization Act required women to be included in clinical trials, progress was slow. By 2023, women made up about half of participants in NIH-funded studies. But only 12% of pharmacokinetic studies - the ones that measure how drugs move through the body - actually analyzed results by sex. That means dosing guidelines for hundreds of medications are still based on how men’s bodies handle them.How Women’s Bodies Process Drugs Differently
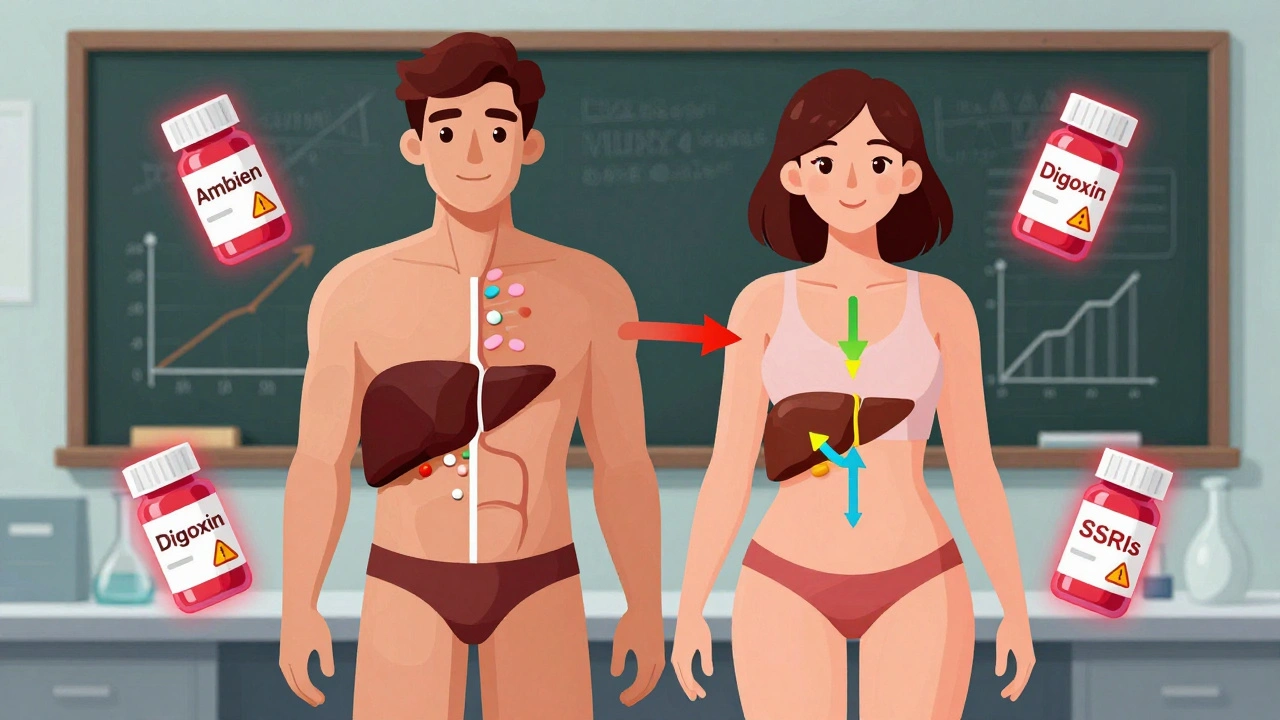
It’s not just about hormones. It’s chemistry, size, and biology. Women have, on average, 40% less of the liver enzyme CYP3A4 than men. That enzyme breaks down about half of all prescription drugs - including antidepressants, statins, and sleeping pills. Less enzyme means drugs stick around longer. That’s why women taking zolpidem (Ambien) were getting groggy the next morning. The FDA finally required a 50% lower dose for women in 2013 - after decades of reports from patients and doctors. Body composition matters too. Women typically have 10-12% more body fat than men. Fat-soluble drugs like diazepam (Valium) get stored in fat tissue and release slowly. That means women keep the drug in their system 20-30% longer than men, even at the same dose. Kidneys work differently, too. Women clear drugs like lithium 22% slower than men. That’s why standard doses can lead to toxicity in women even when they’re taking the exact same amount as men. And hormones? They shift everything. Birth control pills can make the body clear lamotrigine - an epilepsy drug - up to 60% faster. That means women on the pill might need higher doses. But when they stop taking birth control, the dose becomes too high. It’s a constant balancing act.Specific Drugs That Hit Women Harder
Some drugs have clear, documented sex-based risks:- Zolpidem (Ambien): Women metabolize it 50% slower. FDA lowered the recommended dose for women in 2013. After the change, adverse event reports from women dropped by 38%.
- Digoxin: Used for heart failure. Women have 20-30% higher blood levels at standard doses. That raises their risk of toxicity by 40%.
- SSRIs (like sertraline or fluoxetine): Women report 1.5 to 2 times more nausea and dizziness. Men report more sexual side effects - but women suffer more from the physical ones.
- Antipsychotics (like haloperidol): Women are 2.3 times more likely to develop QT prolongation - a heart rhythm problem that can be deadly.
- Sulfamethoxazole (antibiotic): Women have a 47% higher risk of severe skin reactions.
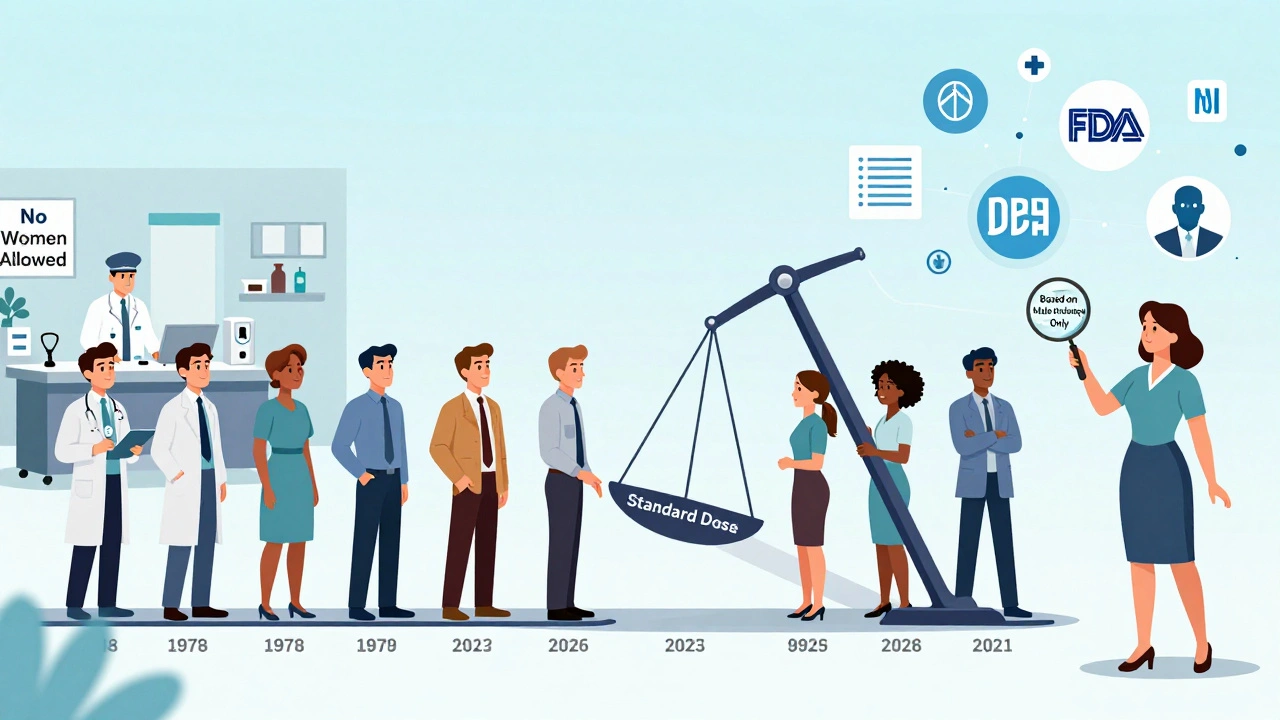
Who’s Taking the Most Medication - and Paying the Price
Women take 59% of all prescription drugs in the U.S. - but they make up only 50.8% of the population. That’s not because they’re sicker. It’s because they’re more likely to be diagnosed with chronic conditions like depression, autoimmune disorders, and chronic pain. They’re also more likely to see a doctor regularly and report symptoms. That reporting bias matters. Dr. Sarah Richardson from Harvard points out that when researchers adjust for how often women take medications, the difference in adverse events shrinks. But that doesn’t mean biology isn’t real. It just means we’re seeing two things at once: biological differences and behavioral patterns. Dr. Janine Austin Clayton from the NIH says both are true: women’s bodies react differently, and they’re also more likely to speak up about side effects. The result? More data on women - but not always better care.The Real-World Impact
A nurse in Sydney told me about a woman who came into the ER after taking a standard dose of ibuprofen for back pain. She ended up with severe stomach bleeding. The woman had been taking the same dose for years. Her doctor never considered her sex. Neither had the pharmacist. That’s not rare. A 2022 survey of 15,000 chronic pain patients found women were more than twice as likely as men to stop taking opioids because of side effects. Nearly two-thirds of those women said they had to lower their dose or quit altogether. On Drugs.com, female users of sertraline (Zoloft) reported severe nausea 68% more often than male users. On Reddit, nurses and pharmacists say they see the same pattern: women come in with dizziness, fatigue, and nausea - often after taking doses that are perfectly fine for men. And it’s expensive. Adverse drug reactions cost the U.S. healthcare system $30 billion a year. Women account for 63-70% of those costs - even though they’re not taking more drugs than men. They’re just more likely to react badly to the same dose.Why Doctors Don’t Know This
A 2022 American Medical Association survey found only 28% of doctors routinely consider sex when prescribing common medications. Two-thirds didn’t even know about the FDA’s 2013 zolpidem dose change for women. Drug labels are the problem. Out of 200 commonly prescribed medications, only 15 have sex-specific dosing instructions. That’s less than 8%. Most labels say “standard dose” - meaning, “dose based on men.” Even when studies show sex differences, it takes 10 to 15 years for guidelines to change. Zolpidem’s sex-based difference was known in 1992. The FDA didn’t act until 2013. That’s two decades of women getting the wrong dose.
What’s Changing - and What’s Not
There’s progress. The FDA launched its “Sex and Gender Roadmap” in 2023, aiming to make sex and gender analysis standard across all drug approvals by 2026. The European Medicines Agency now requires sex-stratified data in Phase III trials. The NIH is funding $12.5 million in research on sex differences at Harvard. The University of California’s JUST Dose study is building AI models to predict safe doses for women based on body size, hormones, and metabolism. Early results show a 40% drop in side effects when sex-specific dosing is used. But here’s the catch: only 32% of cardiovascular drug trials still analyze results by sex. And only 37% of new drug approvals in 2022 included meaningful sex-specific safety data. The market is waking up. Femtech companies focused on women’s pharmacology raised $1.4 billion in 2023. But they’re still a tiny fraction of the $970 billion global pharma industry.What You Can Do
If you’re a woman taking medication:- Ask your doctor: “Was this dose tested on women?”
- Track side effects. Write down when they happen - before or after your period, after starting a new pill, etc.
- If you’re on birth control and taking another drug, ask if it affects your dose. Lamotrigine, for example, needs adjustment.
- Don’t assume a “standard dose” is right for you. If you’re getting side effects others don’t, it might not be you - it might be the dose.
- Check if your drug has sex-specific guidelines. Zolpidem, digoxin, and others do.
- Use the FDA’s Drug Trials Snapshots - they now include sex-disaggregated data for new drugs.
- Don’t wait for labels to change. If the science says women metabolize it slower, start lower.
It’s Not About Gender - It’s About Science
This isn’t about women being “weaker” or men being “tougher.” It’s about biology. And it’s about ignoring that biology for decades. We don’t need separate drugs for men and women. We need better dosing. We need data. We need to stop pretending one size fits all. The fix isn’t complicated. It’s just been ignored.When we start testing drugs on both sexes - and analyzing the results separately - we don’t just protect women. We make medicine better for everyone.
Why do women have more side effects from medications than men?
Women have different body composition, hormone levels, and enzyme activity than men. They typically have less of the liver enzyme CYP3A4, which breaks down many drugs, leading to slower metabolism. They also have higher body fat, which affects how fat-soluble drugs are stored and released. These biological differences mean the same dose can stay in a woman’s system longer and cause stronger side effects. Historical exclusion of women from clinical trials means most dosing guidelines were based on male physiology.
Which medications are known to affect women differently?
Zolpidem (Ambien) is the most well-known - women metabolize it 50% slower, leading the FDA to require a 50% lower dose in 2013. Digoxin causes higher blood concentrations in women, increasing toxicity risk. SSRIs like sertraline cause more nausea and dizziness in women. Antipsychotics like haloperidol lead to 2.3 times more QT prolongation in women. Antibiotics like sulfamethoxazole carry a 47% higher risk of severe skin reactions in women. Birth control pills also alter how drugs like lamotrigine are processed.
Is it because women report side effects more often?
Yes, women are more likely to report symptoms and seek care, which increases the number of recorded side effects. But that doesn’t explain everything. Studies that account for how often women take medications still find biological differences in drug metabolism and response. So both factors matter: women take more drugs, and their bodies process them differently.
Are drug labels updated to reflect sex differences?
Very few are. Out of 200 commonly prescribed medications, only 15 have sex-specific dosing instructions. Most labels still say “standard dose,” meaning based on male physiology. Even after the FDA mandated lower zolpidem doses for women in 2013, most other drugs haven’t changed. It takes 10-15 years for new research to become official guidelines.
What’s being done to fix this problem?
The FDA launched its Sex and Gender Roadmap in 2023 to require sex-specific analysis in all drug approvals by 2026. The European Medicines Agency now requires sex-stratified data in Phase III trials. The NIH is funding $12.5 million in research on sex differences. The University of California’s JUST Dose study is building AI models to predict safer doses for women. But adoption is still uneven - only 32% of cardiovascular trials analyze results by sex.
Should women always take lower doses of medication?
Not always - but they should start lower and adjust based on response. For drugs like zolpidem, digoxin, and lamotrigine, starting with a lower dose is backed by science. But for others, the evidence isn’t clear. The key is to ask: “Has this dose been tested on women?” and monitor side effects closely. Don’t assume a standard dose is right for you.




December 10, 2025 AT 07:34 AM
So let me get this straight - we’ve been giving women the same pill doses as men for 50 years because science was too lazy to include them in trials? And now we’re surprised they’re dropping like flies? 🤦♀️
My grandma took Zoloft for 12 years and blamed herself for the nausea. Turns out, her liver was just doing its job - better than theirs.
Someone should slap a warning label on every prescription: ‘This was tested on a 180lb man who doesn’t menstruate.’
December 11, 2025 AT 21:21 PM
Guys, this is just basic pharmacokinetics and you’re acting like it’s a conspiracy 😅
Women have higher body fat %, lower CYP3A4 expression, slower renal clearance, and hormonal fluctuations - all of which directly impact drug distribution, metabolism, and excretion.
It’s not sexism, it’s biochemistry. Even the NIH admits this. The real issue is that pharma still treats ‘male = default’ as a business model.
And yes, women report more side effects - but that’s because they’re more likely to see a doctor and less likely to be gaslit into thinking it’s ‘just anxiety.’
Also, don’t forget that SSRIs bind more strongly to serotonin transporters in female brains due to estrogen modulation - so nausea isn’t ‘all in their head,’ it’s in their receptors.
And if you think dosing is ‘one size fits all’ - try giving a 120lb woman the same dose of lithium as a 200lb guy. She’ll end up in the ER with tremors and confusion. Been there.
Also, birth control isn’t just ‘a pill’ - it’s a CYP3A4 inducer. Lamotrigine clearance jumps 60%? That’s not a suggestion, that’s a clinical fact. You want to prevent seizures? Adjust the dose. Simple.
And before someone says ‘but men get side effects too’ - yes, but they’re different. Men get sexual dysfunction from SSRIs, women get GI chaos. Both are valid. Both are ignored.
And no, ‘standard dose’ doesn’t mean ‘safe for everyone.’ It means ‘tested on men.’
Also, why do you think women are 70% of adverse drug reaction costs? Because we’re the ones getting the wrong dose. Not because we’re ‘hysterical.’
It’s 2024. We have AI models that can predict sex-based dosing. Why are we still flying blind? Because profit > science. Sad.
Also, the FDA’s 2013 zolpidem change saved lives. Why didn’t they do it in 1993? Politics. Not science.
And if you’re a doctor who doesn’t know this - go read the FDA’s Drug Trials Snapshots. They’re free. And if you’re a patient - ask your prescriber: ‘Was this dose tested on women?’
It’s not complicated. It’s just been neglected. And that’s criminal.
December 13, 2025 AT 20:38 PM
Wow. Another ‘women are fragile’ article. Can we please stop treating biology like a victim narrative?
Men die younger. Men overdose more. Men ignore symptoms until they collapse. So why are we suddenly acting like women are the only ones getting screwed by medicine?
This isn’t sexism - it’s statistics. And you’re cherry-picking data to fit a narrative.
Also, ‘standard dose’ means ‘effective for most.’ Not ‘perfect for every single body.’
Next you’ll be saying we need different insulin doses for left-handed people.
December 14, 2025 AT 18:57 PM
There’s a critical gap here between pharmacokinetics and clinical implementation. Even when sex-specific data exists - like with zolpidem - it takes an average of 12.7 years for guidelines to update due to inertia in medical education and regulatory lag.
Pharmaceutical labeling remains the weakest link: only 7.5% of FDA-approved drugs have sex-specific dosing in their labels, despite 80% of them showing significant pharmacodynamic differences.
And while the JUST Dose initiative is promising, it’s still siloed - no national algorithm integrates sex, BMI, hormonal status, and renal function into e-prescribing systems.
We need CDS alerts in EHRs that flag when a female patient is prescribed a drug with known sex-based metabolic differences - and suggest a 20-50% reduction based on published pharmacokinetic models.
Until then, we’re just repeating the same error: assuming homogeneity in a heterogenous population. It’s not just unethical - it’s statistically indefensible.
December 15, 2025 AT 17:54 PM
Oh wow, a whole article about how women’s bodies work differently than men’s.
Who would’ve thought?
Maybe if we stopped treating women like broken men, we’d stop getting surprised when they react differently to drugs.
Also, ‘standard dose’ is just code for ‘designed for the average white male.’
And yes, I’m salty. I’ve been on the wrong dose of lamotrigine for three years because my doctor didn’t know birth control affects it.
Thanks for the 2013 FDA update. Took long enough.
December 16, 2025 AT 04:46 AM
This is so important. I’m a nurse and I see this every week.
Women come in with dizziness, fatigue, nausea - and they’re told it’s ‘just anxiety’ or ‘you’re overreacting.’
Then we check the dose - and it’s the same as the male patient who took it last week.
It’s not their fault. It’s the system.
Also, I love that the FDA finally changed the Ambien dose. Took them 20 years, but better late than never.
Hope more doctors start paying attention.
December 17, 2025 AT 05:38 AM
As a pharmacist, I can’t tell you how many times I’ve had to call a prescriber because a woman is getting sick on a ‘standard’ dose.
Most don’t even know about the CYP3A4 difference. Or that fat-soluble drugs accumulate differently.
And don’t get me started on SSRIs - women report nausea 2x more, but we still start them at 50mg because ‘that’s what the label says.’
Start at 25mg. Monitor. Titrate. It’s not hard.
Also, if they’re on birth control, always check for drug interactions. Lamotrigine, valproate, SSRIs - all affected.
Simple changes. Huge impact.
Stop pretending biology doesn’t matter.
December 18, 2025 AT 19:07 PM
Big Pharma is hiding the truth. Women’s bodies are being used as test subjects without consent.
They’ve been poisoning us for decades under the guise of ‘science’
And now they want us to trust their ‘roadmaps’?
Wake up. This isn’t about biology - it’s about control.
They don’t want us to know we’re getting the wrong dose because then we’d stop taking their pills.
And the FDA? They’re just corporate puppets.
Also, did you know the 1970s ban on women in trials was pushed by drug companies? They didn’t want the liability of pregnancy lawsuits.
They’ve been lying to us since the 60s.
Don’t trust the system. Fight back.
December 19, 2025 AT 19:08 PM
It is, indeed, a matter of considerable concern that the historical exclusion of female subjects from clinical pharmacokinetic studies has resulted in a persistent and systemic disparity in the safety and efficacy profiles of pharmacological interventions as administered to the female population. The absence of sex-stratified analysis, particularly in early-phase trials, constitutes a significant methodological flaw that undermines the generalizability of therapeutic guidelines. One might reasonably posit that the continued reliance upon male-derived dosing parameters represents not merely an oversight, but a profound failure of translational medical ethics.
December 20, 2025 AT 23:13 PM
so like... women just get more side effects because they're women?
no wonder i always feel weird after taking anything
also why is the label still saying 'standard dose' like its 1985
lmfao
just make the pill smaller for us pls
December 21, 2025 AT 18:20 PM
bro this is wild 😅
i had no idea my mom was on the wrong dose of her anxiety med for 8 years
she thought she was just ‘sensitive’
turns out birth control made her body clear it too fast
then she stopped the pill and got dizzy as hell
why does no one tell you this stuff?
also why is the FDA so slow??
we have self-driving cars but can’t adjust pill doses for women?
smh
December 23, 2025 AT 10:29 AM
Oh great. Another article blaming men’s biology for women’s side effects.
What about the fact that women are prescribed 60% more antidepressants and anxiety meds?
What about the fact that they’re more likely to be diagnosed with ‘chronic pain’ and ‘hysteria’?
Maybe the problem isn’t that the drugs are wrong - maybe the problem is that doctors are overprescribing to women.
And let’s not pretend women don’t report side effects more - they’re socialized to be ‘more in tune with their bodies’ - which means they’re more likely to complain.
Meanwhile, men die from heart attacks because they won’t say ‘my chest hurts.’
This isn’t science. It’s narrative.
December 24, 2025 AT 03:50 AM
Thank you for writing this. I’m from Nigeria and we don’t have much data here - but I’ve seen it firsthand.
My sister took the same dose of amoxicillin as her husband - got a rash, went to the hospital.
They said ‘allergic reaction.’ But he took the same dose and was fine.
Now I ask every doctor: ‘Is this tested on women?’
They look confused. But I keep asking.
Change starts with questions.
December 24, 2025 AT 19:44 PM
Women take more meds. They report more side effects. End of story.
Stop making everything a gender war.
Men get heart attacks. Women get nausea.
Deal with it.
Also, I’m not paying for your ‘sex-specific dosing’
Standard dose works for most.
Stop overcomplicating it.