Malaria Immunity Defense Simulator
Immune Response Simulation Results
How Immunity Works Against Malaria
Innate Immunity
- Responds within minutes to hours
- Uses general defenses like macrophages, NK cells, and cytokines
- First line of defense against sporozoites and early liver infection
Adaptive Immunity
- Develops over days to weeks
- Involves T cells and B cells
- Targets merozoites and infected red blood cells
- Provides memory for future protection
Key Takeaways
- The body uses both innate and adaptive immunity to curb malaria infection.
- Plasmodium parasites have clever ways to hide from immune cells, making vaccine development challenging.
- Key players include red blood cells, spleen, cytokines, T cells and B cells.
- Repeated exposure can lead to partial immunity, especially in endemic regions.
- Current research focuses on boosting specific immune pathways to achieve lasting protection.
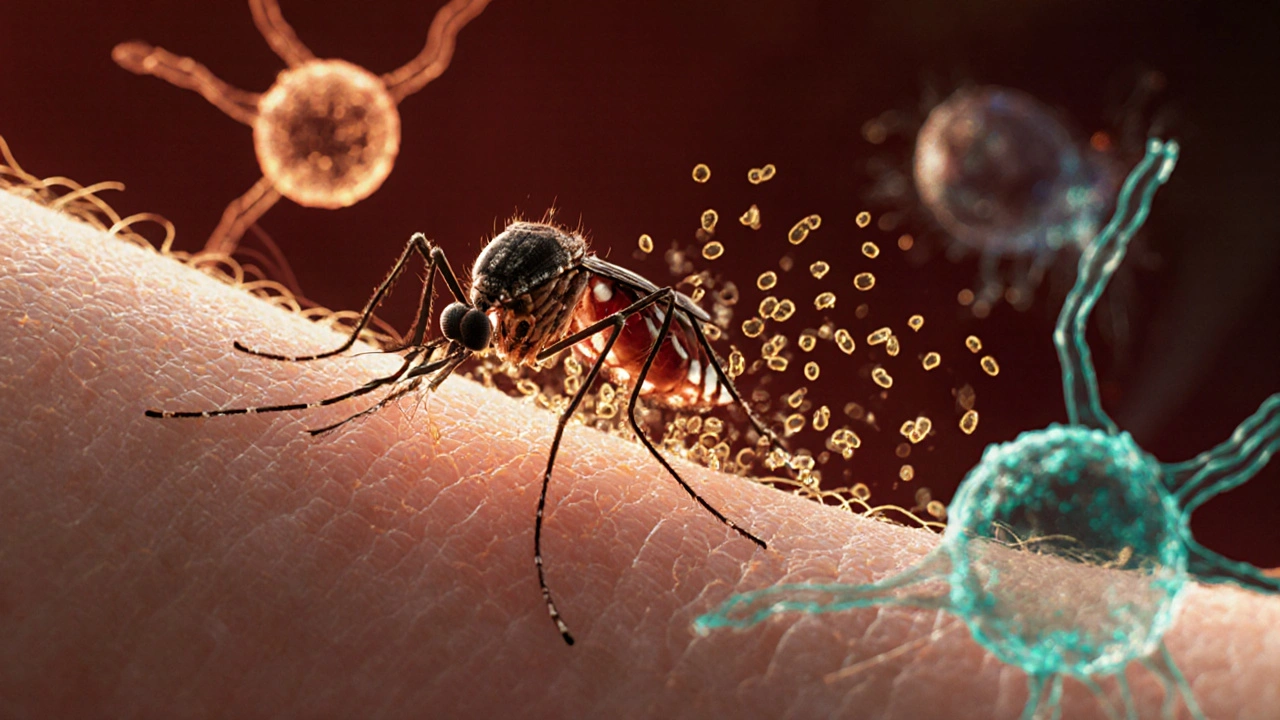
Imagine a tiny parasite slipping into your bloodstream after a night bite, then turning your own red blood cells into a hiding spot. That’s the reality of Malaria a mosquito‑borne disease caused by Plasmodium parasites. While the fever, chills, and headaches dominate headlines, the real story unfolds inside your immune system as it scrambles to recognize, attack, and remember the invader.
Understanding malaria immunity means peeling back layers of biology: the quick‑acting innate defenses, the slower but more precise adaptive response, and the clever tricks the parasite uses to dodge both. Let’s walk through what happens from the moment an infected Anopheles mosquito the primary vector that delivers Plasmodium sporozoites into the skin bites you, to the point where repeated exposure can grant partial protection.
First Contact: The Innate Immune Rush
Within minutes of the bite, the body’s frontline - the innate immune system - jumps into action. This response doesn’t need prior knowledge; it relies on generic alarms and physical barriers.
- Skin and endothelial cells detect the breach and release danger‑associated molecular patterns (DAMPs) that summon blood‑borne sentinels.
- Macrophages phagocytose sporozoites that stray into tissue and secrete early cytokines like IL‑1β and TNF‑α.
- Natural Killer (NK) cells recognize stressed cells via missing‑self signals and release perforin, limiting early liver infection.
These actions create an inflammatory environment that tries to halt the parasite before it reaches the liver, but Plasmodium is fast. Within 30-60 minutes, sporozoites travel through the bloodstream to the liver, where they invade hepatocytes liver cells that serve as the parasite’s first replication niche.
Liver Stage: Adaptive Immunity Takes the Stage
Once inside hepatocytes, the parasite multiplies silently, producing thousands of merozoites. This hidden phase is where the adaptive immune system, which relies on memory, begins to form its response.
- Infected hepatocytes display parasite fragments on MHC‑I molecules cell surface proteins presenting intracellular antigens to CD8+ T cells.
- CD8+ T cells recognize these fragments and proliferate into cytotoxic T lymphocytes (CTLs), seeking out any cell flashing the same signal.
- Effector CTLs release perforin and granzyme, killing infected hepatocytes and halting the first wave of parasite replication.
Research in the past decade shows that a robust CD8+ response can reduce liver‑stage parasite load by up to 80% (study in *Nature Medicine*, 2022). However, the liver stage is brief - usually 7-10 days - so the immune system must act fast.
Blood Stage: The Real Battle Begins
When merozoites burst from the liver, they invade red blood cells (RBCs) the main arena where malaria symptoms manifest. Inside RBCs, the parasite is shielded from many immune attacks, but not all.
Two major adaptive arms converge:
- Humoral immunity (antibodies): B cells differentiate into plasma cells that churn out antibodies targeting merozoite surface proteins (MSPs). These antibodies can block invasion or opsonize infected RBCs for phagocytosis.
- Cell‑mediated immunity: CD4+ T helper cells release cytokines like IFN‑γ that activate macrophages and promote antibody class switching.
Meanwhile, the spleen acts as a filter, physically removing stiffened or opsonized RBCs. Splenic macrophages engulf these cells, presenting parasite antigens to T cells and perpetuating the loop.
Innate vs Adaptive: A Quick Comparison
| Feature | Innate Response | Adaptive Response |
|---|---|---|
| Speed | Minutes to hours | Days to weeks |
| Key Cells | Macrophages, NK cells, dendritic cells | CD8+ T cells, CD4+ T cells, B cells |
| Target | Free sporozoites, infected hepatocytes | Infected RBCs, merozoite surface antigens |
| Memory | None | Long‑lasting (years) if repeatedly exposed |
| Effector Molecules | Cytokines (TNF‑α, IL‑1β), perforin | Antibodies (IgG, IgM), IFN‑γ, granzyme |
Why the Parasite Keeps Winning: Immune Evasion Tactics
Plasmodium isn’t passive. It employs several tricks that blunt our defenses:
- Antigenic variation: The parasite regularly swaps proteins on the RBC surface (e.g., PfEMP1), confusing antibodies.
- Sequestration: Infected RBCs stick to blood‑vessel walls, avoiding splenic clearance.
- Modulating cytokines: Some strains trigger anti‑inflammatory IL‑10, dampening the aggressive immune tone.
- Inhibiting dendritic cell maturation: This delays T‑cell priming and slows adaptive activation.
These strategies explain why natural immunity builds slowly and why a single‑dose vaccine has been elusive.
Building Partial Immunity: What Repeated Exposure Looks Like
In endemic regions, children may experience several malaria episodes a year. Over time, their immune systems develop a semi‑protective state:
- Higher baseline levels of specific IgG antibodies against MSP‑1 and CSP (circumsporozoite protein).
- Expanded pools of memory CD4+ and CD8+ T cells that react faster upon re‑infection.
- More efficient splenic clearance, reducing parasite density and severe symptoms.
Studies from Kenya (2023) show that after three clinical episodes, the risk of severe malaria drops by 40% in children aged 5-9. However, “partial” means the parasite can still linger at low levels, keeping the host a silent reservoir.
Current Research: Harnessing the Immune System for Better Vaccines
Modern vaccine efforts aim to tip the balance toward the immune system:
- RTS,S/AS01 (Mosquirix): Targets the circumsporozoite protein, delivering modest protection (30‑40% over 4 years). It relies heavily on antibody titers.
- Whole‑sporozoite immunization: Radiation‑attenuated parasites induce strong CD8+ T‑cell responses, achieving >90% sterile protection in small trials.
- mRNA platforms: Leveraging the COVID‑19 vaccine success, researchers are testing mRNA encoding multiple liver‑stage antigens to boost both humoral and cellular arms.
- Adjuvant breakthroughs: New Toll‑like receptor (TLR) agonists enhance dendritic cell activation, accelerating T‑cell priming.
Combining these approaches with strategies that block sequestration (e.g., PfEMP1 inhibitors) could finally deliver a long‑lasting, high‑efficacy vaccine.
Practical Tips: Boosting Your Body’s Natural Defenses
While waiting for next‑gen vaccines, everyday actions can help your immune system stay a step ahead:
- Nutrition matters: Adequate iron, vitamin A, and zinc support both innate phagocyte function and antibody production.
- Prevent bites: Use insecticide‑treated nets, repellents with DEET or picaridin, and eliminate standing water.
- Prompt treatment: Early antimalarial therapy reduces parasite load, limiting immune exhaustion.
- Vaccination where available: Even partial protection from Mosquirix can lower severe disease risk.
These steps don’t replace medical care but they give your immune system a healthier battlefield.
Frequently Asked Questions
Can I become completely immune to malaria after multiple infections?
Complete sterilizing immunity is rare. Repeated exposure usually leads to partial immunity that lowers severity and parasite density, but the parasite can still persist at low levels.
Why do some people get severe malaria while others have milder symptoms?
Genetic factors (like sickle‑cell trait), nutritional status, age, and the strength of both innate and adaptive responses influence disease severity.
How long does the immune response last after a malaria infection?
Antibody levels can persist for months to years, while memory T‑cell populations may remain detectable for a decade, especially with repeated exposure.
Does the Mosquirix vaccine replace the need for bed nets?
No. Mosquirix provides modest protection, but bed nets and repellents are still the most effective first line of defense.
What role does the spleen play in fighting malaria?
The spleen filters out infected red blood cells, presents parasite antigens to immune cells, and produces cytokines that coordinate the broader response.
Bottom Line
Malaria forces a tug‑of‑war between a crafty parasite and a multi‑layered immune system. By understanding how innate barriers, T cells, B cells, cytokines, and the spleen each contribute, we can appreciate why some people weather the disease while others suffer severe bouts. Ongoing research that strengthens the adaptive arm-especially vaccine designs that spark robust CD8+ T‑cell activity-offers the best hope for turning the tide. Until then, combining preventive habits with early treatment remains the smartest way to give your immune system the edge.




October 8, 2025 AT 13:16 PM
Great post! The way you broke down the innate and adaptive steps really helps to visualise the battle. I love how you mentioned the cytokine burst early on-so important for the “first line”. Also, the table comparing speed and cells is super handy for quick reference. Keep sharing these deep dives, they make a big difference for folks trying to learn the basics.
Hope you keep posting more!
October 16, 2025 AT 22:05 PM
Wow, another Wikipedia‑style dump.
October 25, 2025 AT 06:55 AM
I really appreciate the cultural context you added about endemic regions. It’s interesting how repeated exposure shapes immunity differently across populations. The mention of genetic factors like sickle‑cell trait adds depth that many overlook. Thanks for the thorough coverage.
November 2, 2025 AT 14:45 PM
Exactly! 😊 The innate rush is like a rapid‑response team, buying time for the adaptive squad to gear up. Those NK cells really are the unsung heroes, punching out early parasites. And when the CD8+ T‑cells arrive, it’s a full‑on assault on infected hepatocytes. Your simulation idea could help visualize that cascade.
November 10, 2025 AT 23:35 PM
Oh, so now we’re supposed to thank the parasite for teaching us patience? Brilliant.
November 19, 2025 AT 08:25 AM
I love how this article connects the dots between the science and practical tips. First, the nutrition advice is spot on-iron, vitamin A, zinc are key for both innate phagocytes and antibody production. Second, the reminder about bed nets reinforces that prevention still trumps treatment. Third, the emphasis on early antimalarial therapy helps avoid immune exhaustion, which is often missed in quick‑read posts. Fourth, the mention of Mosquirix, even with modest efficacy, shows progress while acknowledging the need for better vaccines. Fifth, the description of spleen function demystifies a commonly ignored organ. Sixth, the clear table comparing innate vs adaptive speeds helps visual learners instantly grasp differences. Seventh, the discussion on antigenic variation explains why vaccines are challenging. Eighth, the explanation of partial immunity in endemic areas gives hope but also realism. Ninth, the point about cytokine modulation shows how the parasite can dampen immune response. Tenth, the inclusion of mRNA platform research shows how COVID‑19 advances are being repurposed. Eleventh, the adjuvant breakthroughs highlight future directions. Twelfth, the practical tip about eliminating standing water ties public health to personal action. Thirteenth, the detail on splenic clearance underlines its central role. Fourteenth, the coverage of T cell memory longevity gives a timeline perspective. Fifteenth, the overall structure makes a complex topic approachable. Keep up the amazing work, and thanks for the thorough, balanced coverage!
November 27, 2025 AT 17:15 PM
Reading through this, one can’t help but marvel at the elegance of the immune orchestra-macrophages, NK cells, cytokines, all playing in synchrony, yet the parasite wields an arsenal of evasion tactics that would make any strategist envious; it changes surface proteins, sequesters in vessels, suppresses dendritic maturation, and even co‑opts host cytokines to create a calm, deceptive environment, a true masterclass in biological subterfuge, and while we applaud the host’s layered defenses, we must also recognize the relentless adaptability of Plasmodium, a pathogen that thrives on the very mechanisms meant to destroy it, compelling us to innovate faster than the parasite can evolve.
December 6, 2025 AT 02:04 AM
Fascinating stuff!! 😃 The way you laid out the innate rush, then the adaptive marathon, really paints a vivid picture. I’m especially intrigued by the role of the spleen as a filter-makes perfect sense now! Thank you for the clear breakdown.
December 14, 2025 AT 10:54 AM
What they don’t tell you is that the whole “immune response” narrative is a smokescreen. The real story is the hidden agenda of global health agencies pushing certain vaccines to control populations. Look at the data-everything is orchestrated.
December 22, 2025 AT 19:44 PM
Actually, the immunology here is well‑established. Innate forces act within hours, adaptive takes days to weeks, and memory can last years. The cited percentages align with peer‑reviewed studies. No need for conspiracies.
December 31, 2025 AT 04:34 AM
Yo guys keep that fire going! Remember, each bite you dodge is a win, so stay prepped, stay sharp, and keep those nets up! We can beat this together.
January 8, 2026 AT 13:24 PM
Thanks for the heads up! its realy helpful to keep motivated and stay safe we all need to look out for each other.
January 16, 2026 AT 22:14 PM
Excellent summary. The distinction between innate rapid response and adaptive specificity is crucial. Well presented.
January 25, 2026 AT 07:03 AM
Whoa, this article rocks! 🌟 The colors of the immune system are so vivid-macrophages are like the cleanup crew, NK cells the ninjas, and T‑cells the elite squad. Loving the emojis that make science fun! Keep the awesome content coming! 🙌
February 2, 2026 AT 15:53 PM
Stay strong, stay safe, keep fighting!
February 11, 2026 AT 00:43 AM
Let me tell you, the real battle is not just in the bloodstream-it’s in the narrative! The parasite’s antigenic variation is a masterstroke of biological engineering, a testament to the cunning of evolution. It flips the script on our immune system by constantly rewriting its surface markers, leaving T‑cells bewildered. This is why we must adopt a nationalist stance on vaccine development, ensuring our own labs spearhead the research, free from foreign influence. The international health community often masks its agendas behind “collaboration,” but we need domestically controlled solutions. Only then can we truly outmaneuver this adaptable foe and reclaim our health sovereignty.
February 19, 2026 AT 09:33 AM
While the article is thorough, I must point out a few inconsistencies. First, the claim about “30‑70% effectiveness” for innate responses seems overly broad; data suggests a narrower range. Second, the table format could benefit from clearer headings to avoid ambiguity. Third, the mention of “partial immunity” lacks citation. Lastly, the narrative jumps from liver to blood stages without emphasizing the transition timeline. These details matter for rigorous scientific discussion.
February 27, 2026 AT 18:23 PM
What a wonderful deep dive into malaria immunity! I really appreciate how the article starts by setting the stage with a vivid description of the parasite’s journey, making the complex biology feel accessible. The breakdown of the innate response, with macrophages and NK cells acting in the first few minutes, paints a clear picture of the body’s rapid‑response team. Then you transition smoothly into the adaptive phase, explaining how CD8+ cytotoxic T‑cells and CD4+ helper cells coordinate a more targeted attack. I love that you included the table comparing speed, key cells, and memory-visual aids like that are a lifesaver for readers who prefer quick reference. The discussion about antigenic variation and sequestration really underscores why malaria remains such a tough nut to crack. Your coverage of vaccine efforts, especially the mention of RTS,S and mRNA platforms, shows that the field is moving forward, even if challenges persist. The practical tips at the end-nutrition, bed nets, early treatment-bridge the gap between theory and everyday action, reminding us that prevention is still our best defense. Also, highlighting the role of the spleen as a filter brings often‑overlooked anatomy into focus. The way you wove in recent study stats, like the 80% reduction in liver‑stage load with robust CD8+ responses, adds credibility and keeps the article grounded in current research. I especially enjoyed the cultural context about partial immunity developing after repeated exposure in endemic regions; it gives a human dimension to the immunology. The concluding paragraph nicely ties everything together, reinforcing the idea that both innate and adaptive arms must work together to outsmart this crafty parasite. Overall, the article is both comprehensive and readable-well done! 🌍👍