When you pick up a prescription, you might not realize you’re choosing between two very different kinds of medicine. One is made by just one company with no alternatives. The other is made by dozens, often at a fraction of the cost. Understanding the difference between single-source and multi-source drugs isn’t just about pharmacy jargon-it directly affects your wallet, your health, and your peace of mind.
What Exactly Is a Single-Source Drug?
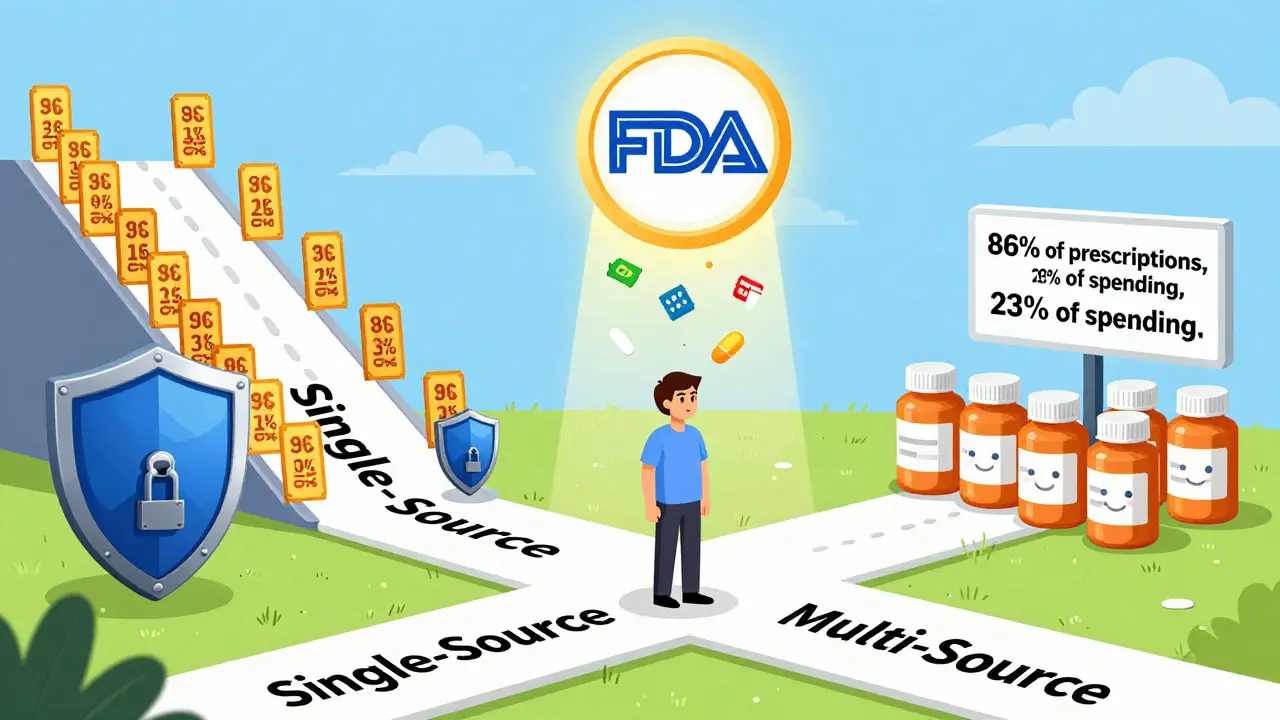
A single-source drug is a medication made by only one manufacturer. Usually, that’s the original brand-name company, and no generic version exists yet. This often happens when a drug is still under patent protection or has special exclusivity rights from the FDA. Think of drugs like Humira before 2023, or newer cancer treatments still locked behind patents. These are the drugs that can cost hundreds or even thousands of dollars per month.Because there’s no competition, the manufacturer sets the price. And they often raise it every year. According to research from the USC Schaeffer Center, when a drug has no generic alternatives, its list price and the rebates it gives to insurers move up together-dollar for dollar. That means the sticker price climbs, but the net cost to insurers doesn’t change much. The problem? Patients still pay based on that high list price, especially if they haven’t met their deductible or are on a plan with high coinsurance.
What Are Multi-Source Drugs?
Multi-source drugs are the opposite. They’re medications that have both a brand-name version and multiple generic versions made by different companies. These are the drugs you see in the “$4 at Walmart” section. Examples include lisinopril for high blood pressure, metformin for diabetes, or atorvastatin for cholesterol. Once the patent expires, other companies can make identical versions-called generics-and sell them at lower prices.The FDA requires these generics to meet strict standards. They must contain the same active ingredient, in the same dose, and work the same way in your body. This is called bioequivalence. The FDA tests them to make sure they’re absorbed in your bloodstream within 80% to 125% of the brand-name drug’s levels. That’s not a wide gap-it’s tight enough to ensure they work just as well.
And the numbers speak for themselves. In 2023, about 86% of all prescriptions filled in the U.S. were for multi-source drugs. But they made up only 23% of total drug spending. That’s the power of competition: more options mean lower prices.
Why Does This Matter for Your Out-of-Pocket Costs?
If you’re paying cash or have a high-deductible plan, the difference between single-source and multi-source drugs can be life-changing.A 2022 Kaiser Family Foundation survey found that patients on single-source drugs were more than twice as likely to skip doses or not fill prescriptions because of cost. Forty-one percent reported skipping medication due to price, compared to just 22% for those on multi-source drugs. The average monthly cost? $587 for single-source drugs versus $132 for multi-source ones.
Patients who switched from brand-name to generic versions reported saving an average of $287 per month, according to a Reddit thread with over 1,200 responses. That’s not pocket change-it’s rent, groceries, or gas.
But here’s the catch: not all generics are treated equally by insurance. Many plans use something called Maximum Allowable Cost (MAC) pricing. That’s the highest amount your insurer will pay for a generic drug. If your pharmacy’s cost is higher than the MAC, you pay the difference. MACs are usually 50-60% below the brand-name’s list price. So even if a generic costs $10, your plan might cap reimbursement at $5. You’re still saving-but not always as much as you think.
Therapeutic Equivalence: Do Generics Really Work the Same?
The FDA says yes. All approved generics must be therapeutically equivalent to the brand. They assign a two-letter code in the Orange Book-like “AB”-to show this. An “A” means it’s bioequivalent. A “B” means it’s not. You’ll never see a “B” on a generic that’s legally sold in the U.S.Still, patients report differences. On Drugs.com, multi-source drugs have an average rating of 4.2 out of 5, while brand-name drugs score 4.5. That small gap isn’t because generics are weaker-it’s because of perception. About 68% of negative reviews for generics mention “inconsistent effectiveness” between different manufacturers. Some patients swear their old generic worked better than the new one they got last month.
That’s not a myth. While the active ingredient is identical, the inactive ingredients-like fillers, dyes, or coatings-can vary. For most people, that doesn’t matter. But for drugs with a narrow therapeutic index (like warfarin, levothyroxine, or some seizure meds), even tiny differences in absorption can cause problems. That’s why pharmacists sometimes flag these when switching generics.
Why Do You Keep Getting Different Generics?
Ever open a pill bottle and see a different color or shape than last time? That’s not a mistake. It’s standard practice.Pharmacy benefit managers (PBMs)-the middlemen between insurers and pharmacies-negotiate deals with generic manufacturers. They pick the cheapest one that meets the MAC. If another company offers a better price next month, your pharmacy switches. According to a 2022 report, 63% of patients on multi-source drugs got switched to a different generic manufacturer within a year.
Most of the time, it’s harmless. But if you’ve had a bad reaction to a specific filler in the past-or if you’re on a sensitive medication-it’s worth asking your pharmacist: “Is this the same manufacturer as last time?” You can also ask for the brand name of the generic (like “Teva” or “Sandoz”) to track consistency.
What About “Single-Source Generics”?
Here’s where it gets tricky. Sometimes, a generic drug is made by only one company-even though the patent expired. How? The original brand company might launch its own generic version, called an “authorized generic.” Or, a PBM might give exclusive rights to one generic maker to keep costs low.This creates a fake monopoly. It looks like a generic, but it’s priced almost like the brand. Truveris’ 2022 analysis found these “single-source generics” aren’t significantly cheaper than the original brand. So don’t assume “generic” always means “cheap.” Always check the price tag.
Insurance Rules and How They Affect You
Your plan’s formulary-the list of drugs it covers-often forces you to try the cheaper option first. This is called step therapy. If you’re prescribed a single-source drug, your insurer might require you to try the generic version first. If that doesn’t work, then they’ll approve the brand.Some plans won’t cover the brand at all unless you prove the generic failed. That means extra paperwork, calls to your doctor, and delays. One 2023 study found patients needed 2-3 pharmacy visits just to understand these rules. Don’t be afraid to ask your pharmacist: “Is there a generic? Will my insurance cover it?”
What Should You Do?
Here’s a simple checklist to take control:- Ask if a generic exists. Don’t assume your prescription is the only option. Pharmacists know the Orange Book codes and can tell you if a cheaper version is available.
- Check your copay. Sometimes, the brand and generic cost the same. That’s rare, but it happens. Always compare prices at the counter.
- Ask about the manufacturer. If you’ve had issues before, request consistency. Say: “I’ve had trouble with this drug before-can I get the same brand of generic?”
- Use the FDA’s website. Visit the FDA’s “Understanding Generic Drugs” page. It’s clear, updated, and free.
- Know your plan’s MAC. If your drug keeps changing, your plan is chasing the lowest price. That’s normal-but you can ask if they’ll lock in one manufacturer for stability.
What’s Changing in 2025?
The FDA is speeding up generic approvals. Under the new GDUFA III rules, they aim to approve generics in 10 months instead of 18-24. That means more single-source drugs will become multi-source faster. Drugs like insulin, biologics, and rare disease treatments are next in line.Also, the Inflation Reduction Act now penalizes drugmakers who raise prices on single-source drugs faster than inflation. That’s a big deal-it could slow down annual price hikes.
But don’t wait for policy changes. Right now, you have power. Ask questions. Compare prices. Don’t let confusion cost you your health.
Are generic drugs really as effective as brand-name drugs?
Yes. The FDA requires all approved generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. They must also prove they’re absorbed into the body at the same rate and to the same extent-within 80-125% of the brand’s levels. For over 90% of medications, this means they work identically. The FDA has monitored this for decades and found no meaningful difference in safety or effectiveness.
Why do I feel different when I switch to a new generic?
You’re not imagining it. While the active ingredient is the same, the inactive ingredients-like binders, dyes, or coatings-can vary between manufacturers. For most people, this doesn’t matter. But for drugs with a narrow therapeutic index-like thyroid meds, seizure drugs, or blood thinners-small differences in absorption can cause noticeable effects. If you notice changes in how you feel after a switch, tell your doctor and pharmacist. You can request the same manufacturer each time.
Can my insurance force me to use a generic?
Yes. Most insurance plans require you to try the generic version first, especially if one exists. This is called step therapy. If the generic doesn’t work for you, your doctor can file an exception request. But if you skip this step, your claim may be denied. Always check your plan’s formulary before filling a prescription.
What’s the difference between a brand-name drug and an authorized generic?
An authorized generic is made by the original brand company but sold under a generic label. It’s chemically identical to the brand, but priced lower. The brand company does this to compete with other generics. Sometimes, authorized generics are cheaper than other generics. But they’re still considered multi-source drugs. The key difference? You’re getting the exact same pill, just without the brand name on the bottle.
How do I find out if my drug is single-source or multi-source?
Ask your pharmacist. They can check the FDA’s Orange Book or their own drug database to tell you. You can also look up your drug on the FDA’s website under “Drug Approvals and Databases.” If you see multiple manufacturers listed, it’s multi-source. If only one company is listed, it’s likely single-source. Don’t rely on the name on the bottle-“generic” doesn’t always mean cheaper if only one maker supplies it.




December 24, 2025 AT 13:07 PM
I used to skip my thyroid meds because the generic kept changing. Then I started asking for the Teva brand specifically-and my TSH levels stabilized. It’s not magic, just consistency. Pharmacists can help if you ask nicely. 🙏
December 25, 2025 AT 00:56 AM
lol the pharma giants are just playing 4d chess with our prescriptions. they got a generic made by one company, call it ‘generic’, charge $200, and we all bow down like it’s a miracle. 🤡
December 25, 2025 AT 08:12 AM
OMG YES!! I switched from brand-name insulin to generic and saved $400/month!! My dog got two extra treats this week 😊💖 #GenericWin
December 25, 2025 AT 20:40 PM
Let’s be real-this entire system is rigged. The FDA’s bioequivalence standards are technically solid, but they ignore real-world variability in absorption, especially for drugs with narrow therapeutic windows. I’ve seen patients crash after a generic switch because their body metabolized it differently due to fillers. And no, it’s not ‘all in their head.’ The system prioritizes cost over individual physiology, and that’s dangerous. You need to track your manufacturer, know your MAC limits, and fight for consistency if your health depends on it. This isn’t just about money-it’s about bodily autonomy.
December 26, 2025 AT 09:46 AM
Just wanted to say-this is the most useful post I’ve read all year. Seriously. I didn’t even know MAC pricing existed. I thought generics were just cheaper. Now I’m asking my pharmacist every time. Thanks for breaking it down so clearly.
December 27, 2025 AT 00:48 AM
My grandma switched to generic metformin and said she felt ‘foggy’ for a week. She went back to the original generic and it was fine. Turns out, the new one had a different dye. She’s 78 and doesn’t care about brand names-just wants to feel normal. That’s all any of us want.
December 27, 2025 AT 02:01 AM
It is fascinating how economic forces manifest in pharmaceutical accessibility. The phenomenon of authorized generics introduces a paradox wherein market competition is simulated, yet monopolistic pricing persists under the guise of affordability.
December 28, 2025 AT 01:47 AM
i think the real issue is we trust the system too much. if the fda says its the same, why do we feel different? maybe the science is incomplete… or maybe they just dont wanna admit that fillers matter. i dont know. but i know i dont trust big pharma anymore.
December 29, 2025 AT 04:44 AM
Wait-so the FDA’s Orange Book is just a marketing tool? And PBMs are secretly working with brand companies to make ‘fake generics’? I just found out my ‘generic’ blood pressure med is made by the same company as the brand. They’re literally playing us. This is a scam.
December 30, 2025 AT 20:25 PM
The structural dynamics of pharmaceutical pricing reveal a dissonance between regulatory assurances and consumer experience. While bioequivalence is statistically validated, the phenomenological impact of excipient variation remains underappreciated in clinical policy. A patient’s subjective response is not anecdotal-it is data.
January 1, 2026 AT 09:48 AM
MAC pricing thresholds are functionally arbitrary and create perverse incentives for PBMs to prioritize cost over therapeutic continuity. The absence of longitudinal pharmacokinetic tracking for generic switches represents a critical gap in post-market surveillance. This is not healthcare-it’s actuarial risk arbitrage.
January 2, 2026 AT 07:01 AM
They’re not just selling drugs-they’re selling control. Every time you get a new generic, it’s a test. Are you obedient? Do you take whatever they give you? Or do you fight? The system wants you passive. Don’t be. Ask for the manufacturer. Demand consistency. Or you’re just another statistic.