When you pick up a prescription, you might see two options on the label: the name you recognize from TV ads, or a simpler, cheaper version with a different color and shape. It’s not a trick. It’s not a downgrade. It’s a generic drug - and it’s just as effective as the brand-name version in nearly every case.
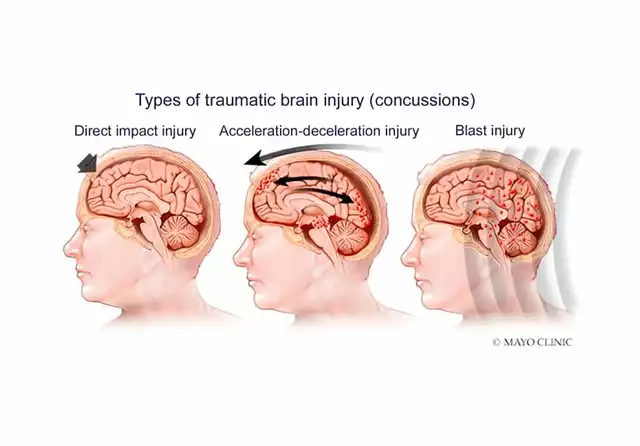
What Exactly Is a Generic Drug?
A generic drug is the same as its brand-name counterpart in active ingredient, dosage, safety, strength, and how it works in your body. The only differences? The name, the shape, the color, and the price. Generics become available after the original drug’s patent expires - usually 20 years after it’s first filed. That’s when other companies can legally make the same medicine without paying for the original research. The U.S. Food and Drug Administration (FDA) requires generics to prove they’re bioequivalent. That means they deliver the same amount of active ingredient into your bloodstream at the same rate as the brand. The FDA’s standard? The 90% confidence interval for absorption must fall between 80% and 125% of the brand. In real-world testing, the difference is usually less than 4%. That’s not a gap - it’s a rounding error.Why Are Generics So Much Cheaper?
Brand-name drugs cost a lot because the company had to pay for years of research, clinical trials, marketing, and patent protection. Generics skip all that. They don’t need to repeat expensive safety studies because the original drug already proved it’s safe. All they need to show is that their version works the same way. The result? Generics cost 80-85% less on average. Take sertraline (the generic for Zoloft): a 30-day supply can cost $4 instead of $400. Atorvastatin (generic Lipitor) runs about $0.10 per pill versus $4.50 for the brand. Over the past decade, generics saved the U.S. healthcare system more than $1.67 trillion. That’s $265 saved per person each year.Are Generics Really Just as Safe and Effective?
Yes - and the data backs it up. A 2019 meta-analysis in JAMA Internal Medicine reviewed 47 studies with nearly 10,000 patients. It found that generics performed identically to brand-name drugs in 98.5% of cases across heart disease, depression, diabetes, and high blood pressure. The FDA holds both generic and brand manufacturers to the same quality standards. Both must meet strict rules for purity, stability, and manufacturing. Inspections in 2022 showed generic manufacturers met compliance standards 98.7% of the time - almost the same as brand-name makers at 99.1%. Even patients who are skeptical often change their minds. A 2022 survey by the American Pharmacists Association found that 68% of patients worried generics wouldn’t work. But after talking to their pharmacist and learning about FDA rules, 89% kept using them. And adherence - meaning taking the medicine as prescribed - went up by 22% because people could actually afford it.
When Might You Still Want the Brand?
There are a few exceptions. For drugs with a narrow therapeutic index - meaning the difference between a helpful dose and a dangerous one is very small - even tiny changes can matter. That’s why some states require special rules for drugs like:- Levothyroxine (for thyroid disease)
- Warfarin (a blood thinner)
- Phenytoin and carbamazepine (for seizures)
Why Do Generics Look So Different?
The law doesn’t let generic manufacturers copy the exact look of a brand-name drug. That’s why your generic pill might be blue instead of yellow, or oval instead of round. It’s also why the name on the pill is different - usually just the chemical name, like “sertraline” instead of “Zoloft.” This causes confusion. A 2023 study in Patient Education and Counseling found that 27% of patients on Reddit and other forums reported anxiety when their pill changed color or shape. They worried it was a different drug. But it’s not. The FDA requires all pills to be marked with unique imprints so you can look them up in the Drugs@FDA database if you’re unsure.What About Those Bad Reviews?
You’ve probably seen online complaints: “Generic Wellbutrin didn’t work for me.” “My seizures came back after switching.” Some of these are real. In 2012, the FDA warned that certain generic versions of extended-release bupropion (Wellbutrin XL) didn’t match the brand’s release pattern. That led to reformulations. Today, the approved generics are fine. But here’s the catch: people are more likely to notice and report problems after switching. If you’ve been on a brand for years and suddenly feel “off” after switching to a generic, it’s easy to blame the drug. But it could be stress, sleep, diet, or even the placebo effect wearing off. The bottom line? Most complaints aren’t about the medicine - they’re about change.
How to Make the Smart Choice
You don’t need to be a pharmacist to make the right call. Here’s what to do:- Ask your pharmacist: “Is there a generic for this? How much will it save me?”
- Check GoodRx or your pharmacy’s price list. Sometimes the brand is cheaper with a coupon.
- If you’re on a narrow therapeutic index drug, ask your doctor if you should stick with one version.
- If your pill looks different, use the Drugs@FDA tool to confirm it’s the same medicine.
- Don’t switch brands or generics without talking to your doctor - especially for seizure meds, blood thinners, or thyroid drugs.
What’s Changing in 2026?
The Inflation Reduction Act of 2022 is starting to take effect. Starting in 2026, Medicare will negotiate prices for 10 high-cost brand-name drugs. That’s likely to push even more patients toward generics - and force manufacturers to lower prices to stay competitive. Meanwhile, the FDA is speeding up approval for complex generics - things like inhalers, eye drops, and injectables - which have been slow to come to market. By 2027, generics are expected to make up 93% of all prescriptions filled in the U.S.Final Thought: Cost Isn’t the Only Factor - But It’s Often the Most Important One
Brand-name drugs aren’t better. They’re just older, more expensive, and more familiar. For most people, generics are the smarter, safer, and more affordable choice. The science is clear. The savings are real. And the only thing standing in the way? Fear - and misinformation. If your doctor says you can switch, and your pharmacist says it’s safe - go for it. Your wallet, and your health, will thank you.Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove bioequivalence - meaning they deliver the same amount of medicine into your bloodstream at the same rate. Studies show generics work just as well in 98.5% of cases across common conditions like high blood pressure, depression, and diabetes.
Why do generic drugs look different?
U.S. law prohibits generic manufacturers from copying the exact appearance of brand-name drugs. That’s why generics may have a different color, shape, or imprint. These differences are only in inactive ingredients like dyes or fillers - they don’t affect how the medicine works. You can always check the pill’s imprint using the FDA’s Drugs@FDA database to confirm it’s the correct medication.
Can switching to a generic drug cause side effects?
Side effects from switching are rare. Most people feel no difference. But in some cases - especially with drugs that have a narrow therapeutic index like levothyroxine or warfarin - switching between different generic versions can cause small changes in blood levels. That’s why doctors may recommend sticking with one manufacturer. If you notice new symptoms after switching, talk to your doctor or pharmacist.
Are there any drugs where I should avoid generics?
For most medications, generics are safe. But for drugs with a narrow therapeutic index - such as warfarin, levothyroxine, phenytoin, and some anti-seizure medications - your doctor may recommend sticking with one version. Complex delivery systems like inhalers, patches, or extended-release capsules can also be tricky if the device changes. Always ask your pharmacist or doctor before switching.
How do I know if my pharmacy is giving me a generic?
The prescription label will list the generic name (e.g., “atorvastatin”) instead of the brand name (e.g., “Lipitor”). You can also ask the pharmacist directly. Most pharmacies will automatically fill with a generic unless your doctor writes “dispense as written” or you specifically request the brand. You can also check pricing - generics are almost always cheaper.
Can I switch back to the brand if the generic doesn’t work for me?
Yes. If you feel the generic isn’t working as well - or if you have side effects - talk to your doctor. They can write a prescription for the brand-name drug, or help you try a different generic manufacturer. Sometimes the issue isn’t the drug itself, but a change in inactive ingredients or even psychological factors. Your pharmacist can also help identify if you’re getting a different version than before.




January 4, 2026 AT 04:07 AM
Wow, this is such a needed post! I used to freak out every time my pill changed color-thought I was getting fake meds or something. Then I learned about the FDA’s bioequivalence rules, and now I’m all in on generics. Saved me like $200/month on my blood pressure med. My wallet and my sanity thank you!
January 5, 2026 AT 09:04 AM
How quaint. You’re parroting FDA talking points like a corporate bot. The reality? Bioequivalence is a statistical mirage. The 80–125% confidence interval is a regulatory loophole disguised as science. Real pharmacokinetics don’t care about averages-they care about individual variance. And yet, you casually dismiss the clinical anecdotes that contradict your orthodoxy. Pathetic.
January 6, 2026 AT 10:33 AM
Okay, but let’s get real for a sec-why do generics look so weird? Like, I get the law, but why not just use the same damn color? It’s not like the dye affects absorption. And why do some generics have that weird chalky taste? I swear, my generic metformin tastes like a science fair project gone wrong. Meanwhile, the brand version? Smooth. Like swallowing a vitamin. Is that just placebo? Or are the fillers actually different? Asking for a friend who’s now suspicious of everything.
January 8, 2026 AT 09:09 AM
Have you considered that the reason people feel different on generics isn’t just psychological? What if the inactive ingredients-like lactose or dyes-are triggering subtle immune responses? I’ve seen patients with autoimmune conditions crash after switching. No one talks about this. The FDA doesn’t require comparative testing of excipients. That’s not oversight-it’s negligence.
January 9, 2026 AT 17:40 PM
Thanks for this. In Nigeria, we don’t even have access to generics most times. When we do, they’re often counterfeit. So reading this makes me hopeful. Maybe one day we’ll have the same trust in meds as you do. 🙏
January 10, 2026 AT 08:07 AM
Oh wow, another ‘generics are just as good’ sermon. How original. Let me guess-you also think vaccines are fine if they’re ‘bioequivalent’ and buy store-brand diapers because ‘they’re the same.’
January 10, 2026 AT 15:14 PM
Excellent breakdown. I’ve worked in pharmacy for 18 years, and I can confirm: 99% of the time, generics are indistinguishable. The exceptions? Levothyroxine, warfarin, and the occasional extended-release capsule. But even then, if you stick with one manufacturer, it’s fine. The real issue? Patients panic when the pill looks different. We need better patient education-not fear-mongering.
January 10, 2026 AT 22:09 PM
Ugh. This is the same tired, corporate-approved narrative. You know who profits from generics? Big Pharma’s subsidiaries. The same companies that make the brand names. They own the generic manufacturers. It’s all a money laundering scheme disguised as ‘affordability.’ You’re being played. The FDA? Bought and paid for.
January 12, 2026 AT 19:21 PM
Generic Wellbutrin gave me anxiety. I switched back. Done. End of story. Also, why is everyone so obsessed with stats? I’m not a lab rat.
January 14, 2026 AT 08:47 AM
While the data presented is statistically sound, it is fundamentally flawed in its epistemological framing. The assumption that bioequivalence equates to therapeutic equivalence ignores the ontological reality of individual physiological variance. Furthermore, the FDA’s regulatory framework is predicated on population-level norms, which are epistemologically insufficient for personalized medicine. One cannot reduce pharmacodynamics to a confidence interval.
January 14, 2026 AT 17:41 PM
THEY’RE MAKING US SWITCH TO CHINA PILLS!! 🇨🇳💀 You think they care about your health? NO. They care about the 2026 Medicare price cap. This is a socialist takeover of your medicine! I saw a video-generic pills are made in basements with rat hair and lead paint! I checked the imprint-my levothyroxine says ‘M 10’-that’s not even a real code! THEY’RE LYING TO US!!
January 15, 2026 AT 10:09 AM
Man, I’m from the South, and we don’t trust anything that doesn’t come in a fancy blue pill. But my grandma switched to generic Lipitor and now she’s hiking every morning. She says, ‘If it keeps me alive and doesn’t cost me my Social Security check, I ain’t complainin’.’
January 17, 2026 AT 07:13 AM
My doctor told me to switch to generic sertraline. I did. I felt like a zombie for two weeks. Then I switched back. The brand worked. The generic didn’t. Case closed. Don’t tell me about ‘bioequivalence’-my brain knows the difference.